Units of pressure are often expressed as P FA eg. Measures absolute gas concentration but is calibrated to read out in relative units a 1 kPa pressure increase at sea level results in an apparent O2 increase of 0207 000987 2095.

Partial Pressure Easy Science Easy Science Chemical Changes Pressure
The unit of 1 atmosphere used to describe the pressure of a gas is equal to _____ asked Sep 16 2016 in Chemistry by Marra.

. A 23 atm to torr b 47 x 10-2 atm to millimeters of mercury c 248 psi to millimeters of mercury d 3284 in. You are misreading it it is mm of Hg. Select all that apply.
Pressure is a scalar quantity. Where P is pressure F is force and A is area. There are many units which are used to measure the pressure.
One pascal is a small pressure. It may help to consider pressure of a gas. A pascal is a very small amount of pressure so the kilopascal kPa is more commonly used.
Pressure is a derived unit generally expressed according to the units of the equation. 1 atm 101325 Pa 760 torr. The relationships between these pressure units are.
Answered Sep 16 2016 by Enka31. The unit of 1 atmosphere used to describe the pressure of a gas is equal to 760 mmHg O 1 mmHg O 200 mmHg O 600 mmHg O100 mmHg. Temperature volume amount and pressure.
Kenny6666 7 1 year ago 3 0 Answer is 1 atm 760 mm Hg. Units used to describe absolute and relative gas concentration measurements. A manometer is a simple pressure measuring device which utilizes mercury and atmospheric pressure in combination to determine the pressure of a gaseous system.
Pressure is force per unit area of surface. This means millimeters of Mercury. In many cases it is more convenient to use units of kilopascal 1 kPa 1000 Pa or bar 1 bar 100000 Pa.
What does mm of hydrogen gas mean. The unit of 1 atmosphere used to describe the pressure of a gas is equal to 760 mm hg. Convert each measurement to atm.
Hg to torr 28. The pressure of a gas may be expressed in the SI unit of pascal or kilopascal as well as in many other units including torr atmosphere and bar. -In an open-end manometer the gas pressure pushes on the Hg surface on one arm of the U tube and atmospheric pressure gas pushes on the other.
In many cases it is more convenient to use units of kilopascal 1 kPa 1000 Pa or bar 1 bar 100000 Pa. Other units used to describe gas pressure are the atmosphere atm torr millimeter of mercury mmHg and bar. The SI unit for pressure is the pascal Pa defined as 1 newton per square meter Nm 2.
Which of the following options correctly describe the gas constant R. Four quantities must be known for a complete physical description of a sample of a gas. Examples are atm Pascal Pa torr mm Hg and bar.
Absolute Amount of Gas Relative Amount of Gas moles of O2 per unit. The SI unit of pressure is the pascal Pa with 1 Pa 1 Nm 2 where N is the newton a unit of force defined as 1 kg ms 2. The pressure exerted by an object is proportional to the force it exerts and inversely proportional to the area.
A 1 mmHg B 100 mmHg C 200 mmHg D 600 mmHg E 760 mmHg. P F A. Instruments used to measure pressure are called pressure gauges or vacuum gauges.
A standardized prefix system indicates fractions and multiples of. Meaning it has a magnitude but not a direction. -In an closed-end manometer the gas pressure is equal to the difference in column heights in the two arms of a U tube.
The unit of pressure in the SI system is the pascal Pa defined as a force of one Newton per square meter. Pressure volume and temperature and moles of gas are the four principal variables to describe a gas for example see related questions on Ideal Gas Law and others. Pounds per square inch psi dynes per square inch or Newtons N per square meter Nm² Definition of Pressure.
Consider a manometer tube filled with mercury attached to a vessel containing a sample of gas. The unit of 1 atmosphere used to describe the pressure of a gas is equal to 760 mm hg. The SI unit of pressure is the pascal Pa with 1 Pa 1 Nm 2 where N is the newton a unit of force defined as 1 kg ms 2.
The pressure is defined as the force per unit area which is perpendicular to the surface. - Answers Standard atmospheric pressure at sea level is equal to. Other gas pressures can be measured using one of several types of manometers.
12 The unit of 1 atmosphere used to describe the pressure of a gas is equal to 12 the unit of 1 atmosphere used to describe the School Gadsden State Community College. In a Boyles law calculation any units of pressure and volume may be used as long as both the initial and final condition units match. Thus the formula is often expressed as P FA.
Atmospheric pressure is measured using a barometer. A 1277 mm Hg b 238 X 105 Pa c 127 psi d 455 torr 26 25. This may seem confusing since its usually obvious the force has direction.
One pascal is a small pressure. The conversion between atm Pa and torr is as follows. The standard units are.
At STP the density of argon is _____. The accepted SI unit for gas pressure is the pascal Pa. Unit of 1 atmosphere used to describe the pressure of gas.
101325 Pa 10 Pa 10 N m2 101325 kPa 101325 millibar.

Navier Stokes Equations Explain The Motion Of Fluids Gases And Liquids The Equation Is Derived When Yo Equations Fluid Mechanics Engineering Fluid Dynamics
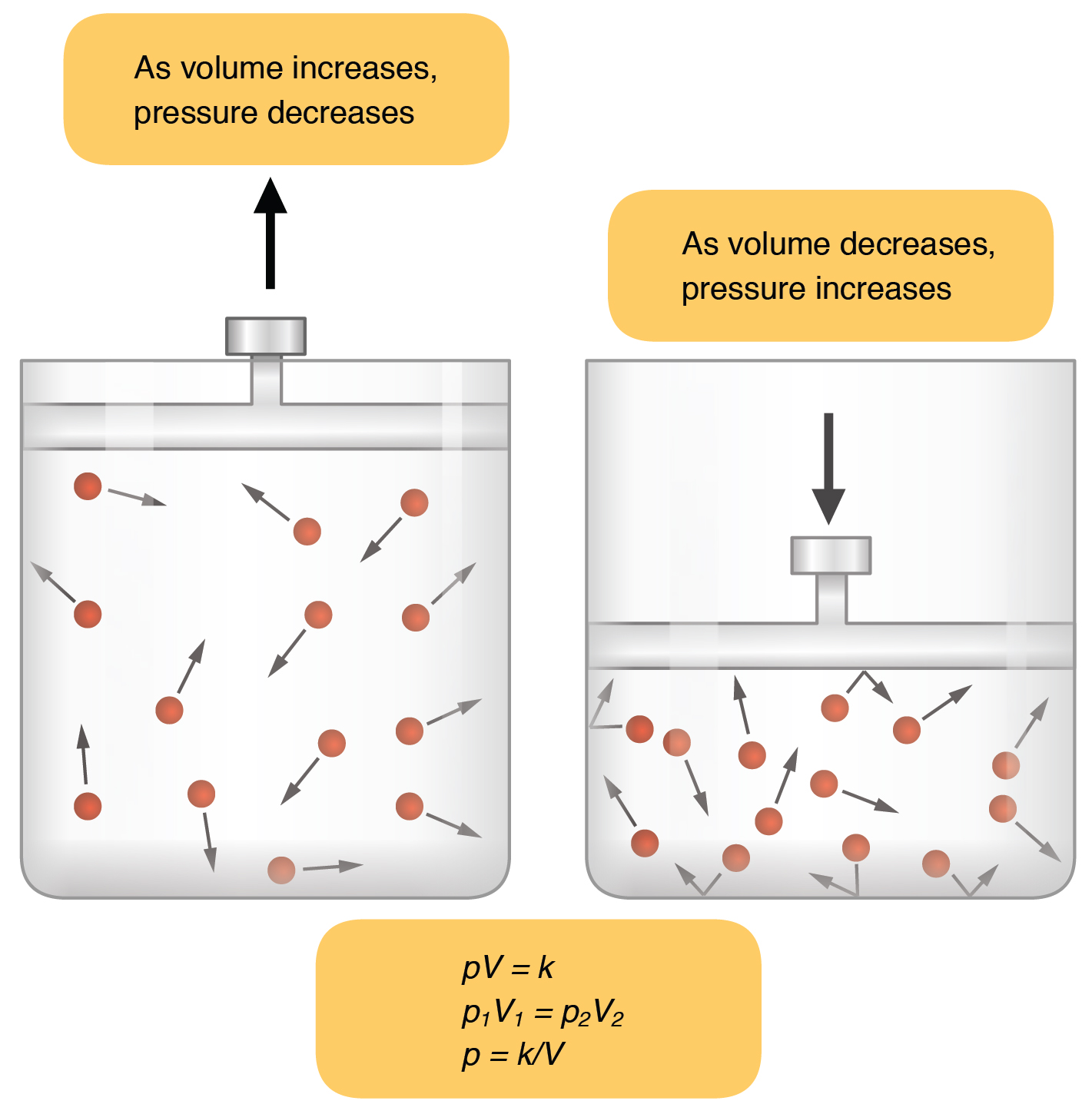
Scientific Accomplishments Robert Boyle Is Most Known For His Creation Of Boyle S Law In Chemistry Which St Chemistry Classroom Chemistry Lessons Boyle S Law

Units Of Pressure Chemistry Notes Chemistry Worksheets Chemistry Notes Teaching Chemistry

Ideal Gas Constant Easy Science Gas Constant Ideal Gas Law Chemical Changes

14 4 Measuring Pressure Physics Libretexts Physics And Mathematics Learn Physics Fluid Mechanics

R410a R22 R134a R600a R32 R404a R417 R290 R407 R290 All Gas Information R Refrigeration And Air Conditioning Air Conditioner Repair Air Conditioner Maintenance
Pin By Jessica Joyce On Ideal Gas Law Ideal Gas Law Gas Laws Chemistry Chemistry Lessons

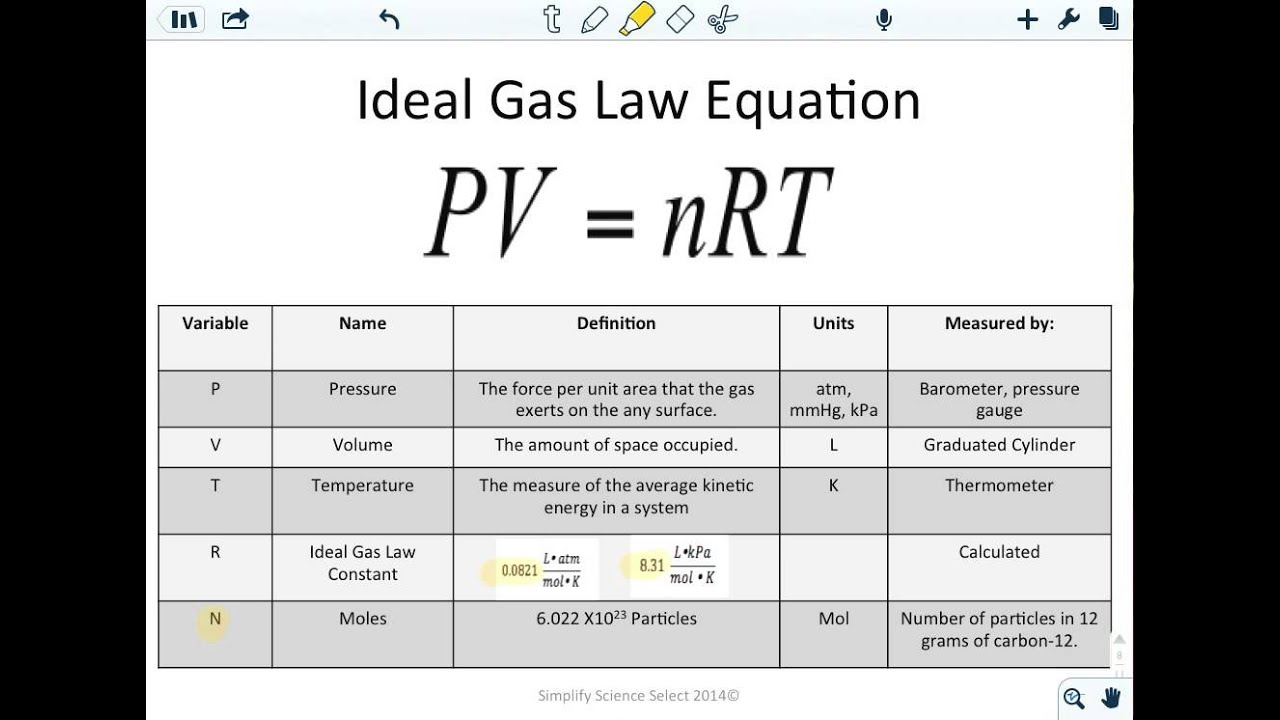
Ideal Gas Law Notes Ideal Gas Law Law Notes High School Chemistry

0 Comments